JOINT TRANSNATIONAL CALL 2021
“MULTIDISCIPLINARY RESEARCH PROJECTS ON PERSONALISED MEDICINE – DEVELOPMENT OF CLINICAL SUPPORT TOOLS FOR PERSONALISED MEDICINE IMPLEMENTATION”
The call is closed.
ERA PerMed is an ERA-NET Cofund, supported by 32 partners of 23 countries and cofunded by the European Commission (EC). To align national research strategies, promote excellence, reinforce the competitiveness of European players in Personalised Medicine (PM), and enhance the European collaboration with non-EU countries, 30 funding organisations have agreed to launch the fourth Joint Transnational Call for collaborative innovative research projects in PM. This represents the third additional call non-cofunded by the EC. The funding organisations participating in this call particularly wish to promote innovative interdisciplinary collaboration and to encourage translational research proposals. The available budget for this call is 24 Mio € (approx.).
Opening of online submission tool: 14 December 2020
Submission deadline for pre-proposals: 4 March 2021 (17:00 CET)
Opening of online submission tool for full-proposals: 13 May 2021
Submission deadline for invited full-proposals: 17 June 2021 (17:00 CET)
Electronic submission website
Electronic proposal submission is mandatory on PT-Outline. Research project consortia who intend to submit a transnational proposal should register as soon as possible, by clicking on “Sign up” and follow further instructions.
Contact persons for the Joint Call Secretariat (JCS)
The ERA PerMed JCS is hosted by the Italian Ministry of Health (It-MoH):
Viale Ribotta, 5 Roma, ITALY
With the support of the Fondazione Regionale per la Ricerca Biomedica, (FRRB), Lombardy (Italy).
Maria Jose Ruiz Alvarez, Monica Paganelli
Phone: +39 06 5994 3214 / 2408
Email: healthresearch@sanita.it
CALL Documents
The call text and all additional documents can be downloaded below or on the online submission tool:
Call Text (updated 14-04-2021)
Guidelines for Applicants (updated 14-04-2021)
Pre-Proposal form (updated 14-12-2020)
Full-Proposal form (updated 21/05/2021)
Annex template for exploratory clinical studies (updated 29-12-2020)
Contact details for countries participating in call 2021 (updated 14-04-2021)
ITALY (IT-MoH): Pre-eligibility check form for IT-MoH proposals (to be sent to IT-MoH at least 10 working days before the JTC submission deadline).
ITALY (FRRB): Pre-eligibility check form for FRRB proposals (to be sent to FRRB at least 10 working days before the JTC submission deadline) and budget tool as support for FRRB applicants.
ITALY (TuscReg): Pre-eligibility check form for TuscReg proposals and budget compilation form for TuscReg proposals (both to be sent to TuscReg at least 10 working days before the JTC submission deadline).
SPAIN (ISCIII and FCAECC): Additional application form for partners requesting funds from ISCIII and FCAECC (to be sent as annex of BOTH pre-proposal and full proposal submission form via the online submission tool).
Data Management Plan (DMP) – will be requested ONLY from funded proposals
AIMS OF THE CALL
With its fourth transnational call (non-cofunded by the EC), ERA PerMed fosters research and innovation activities that build close linkages between clinical research, computer science/medical informatics and research on ethical, legal and social aspects (ELSA) in the field of PM. This implies a wide range of multidisciplinary activities brought together by different stakeholders from academia, clinical/public health research and private partners such as small and medium-sized enterprises (SMEs), policy makers, regulatory/health technology assessment (HTA) agencies and patients/patient organisations.
The overarching goal is to improve disease prevention and disease management, based on broader and more efficiently characterised and defined patient stratification, diagnostics and tailored treatment/prevention protocols for both patients and individuals at risk of disease. Early involvement of regulatory authorities and close interaction with the different key contributors along the value chain should be included right from the project development phase to bridge the gap between first discoveries or inventions to market access.
Research proposals submitted under this call are expected to demonstrate the applicability of project outcomes to clinical practice and to combine clinical research with data technologies. This could be the development and application of clinical decision support tools by using artificial intelligence (AI) systems approaches, including machine learning technologies. The clinical relevance of the proposed PM approach needs to be convincingly demonstrated. Moreover, proposals must include research on ethical, legal and social aspects.
As Personalised Medicine is non-disease-specific, but rather an overall approach that can be adopted and adapted to a multiplicity of medical conditions, research projects in every disease entity are encouraged.
The involvement of partners with the respective expertise in the consortium is required.
Additionally, projects may include pre-clinical research as a prerequisite for the implementation of a PM approach into clinical practice. Multilevel health economic assessment is also considered to be important for facilitating the translation of PM approaches to healthcare and can be included in the work plan.
The overall objectives of the call are to:
The overall objectives of the call are to:
Support translational and transnational research projects in the field of PM;
Encourage and enable interdisciplinary collaborations towards the implementation of PM, combining clinical research with bio-informatics components and research on relevant ethical, legal and social aspects. Additionally, pre-clinical and health economic research can be included if the added value is outlined;
Encourage collaboration between academia (research teams from universities, higher education institutions, public research institutions, research centres), clinical/public health research (research teams from hospital/ public health, healthcare settings and other healthcare organisations), private partnersg. SMEs[1] (small and medium-sized enterprises) as well as policy makers, regulatory/HTA agencies and patient representative organisations.
[1] https://ec.europa.eu/growth/smes/business-friendly-environment/sme-definition_en
SCOPE OF THE CALL
Proposals must be interdisciplinary and clearly demonstrate the potential impact in PM as well as the added value of transnational collaboration.
The JTC2021 is constructed around the following three research areas in order to ensure the development of specific PM approaches, taking into account the major aspects for their successful implementation in the health systems: (1) “Translating Basic to Clinical Research and Beyond”, (2) “Data and Information and Communication Technology (ICT)” and (3) “Research towards Responsible Implementation in Healthcare”:
Each proposal MUST address the modules 1B “Clinical Research”, 2 “Towards Application in Healthcare” and 3B “Ethical, Legal and Social Aspects”. Inclusion of modules 1A “Pre-clinical Research” and 3A “Health Economic Research” is optional. Their added value to the proposal and the mandatory modules has to be clearly described.
GENERAL (eligibility) CONDITIONS FOR APPLICATION
Joint research proposals may be submitted by applicants belonging to one of the following categories (A, B and/or C), if eligible according to their respective regional/national funding organisation’s regulations for research funding:
Academia (research teams working in universities, other higher education institutions)or research institutes;
Clinical/public health sector (research teams working in hospitals/public health and/or other health care settings and health organisations). Participation of clinicians (e.g. medical doctors, nurses) in the research teams is encouraged;
(Industry) Private partners, e.g. SMEs[2](small and medium-size enterprises).
Although proposals will be submitted jointly by research groups from several regions/countries, research groups will be funded by the respective funding organisation of the region/country from which they have applied. Applicants are therefore subject to the eligibility criteria of their respective funding organisations. Applicants are strongly advised to contact their regional/national representatives of the participating relevant funding organisation as soon as possible in order to confirm their eligibility (see also “Contact details of participating members”).
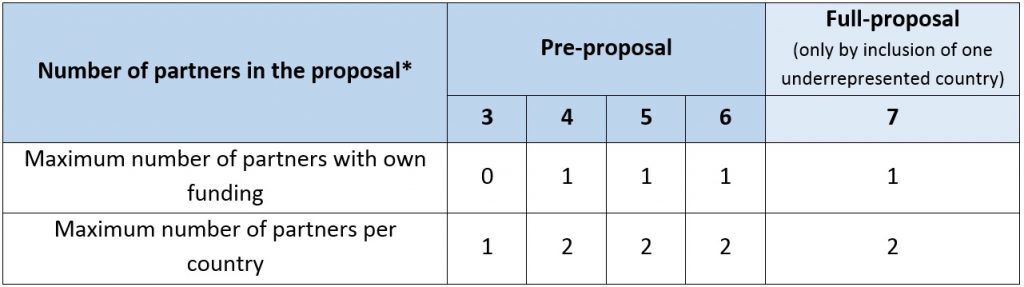
Only transnational projects will be funded. Each consortium submitting a proposal must involve at least three partners eligible for funding coming from three different countrieswhose funders participate in the call. All three legal entities must be independent of each other. At least two partners of the minimum three eligible project partners of the consortium must be from two different EU Member States or Associated Countries.